Background
Industry sponsorship bias is shorthand for a host of ways in which studies get skewed—in the design, conduct and/or publication of research–in order to promote commercial interests.
Mechanisms include, but are not limited to: posing a research question such that the answer is true but misleading; choosing unrepresentative study populations; administering a competitors drug at a non-optimal dose (in comparator trials); questionable choices made while analysing the data; non-publication of statistically nonsignificant results; selective reporting of outcomes; and multiple publication of positive results.
Industry sponsorship bias refers to the tendency of a scientific study to support the interests of the study’s financial sponsor. This bias is also referred to as funding bias, sponsorship bias, funding outcome bias, funding publication bias, and funding effect. (Holman 2018)
Example
Statins are prescribed to reduce cholesterol which in turn is intended to reduce mortality from coronary events. At the time the review by (Bero 2007) was conducted, there were multiple statins on the market produced by a number of competing companies.
Researchers identified 95 RCTs in which multiple statins were compared
or a statin was compared to an older treatment. Statistical
comparisons between drugs were classified as “favourable” to statins
if the newer drug outperformed the competitor, “inconclusive” if the
results were not statistically significant, and “unfavourable” if the
older competitor drug was superior and the result was statistically
significant.
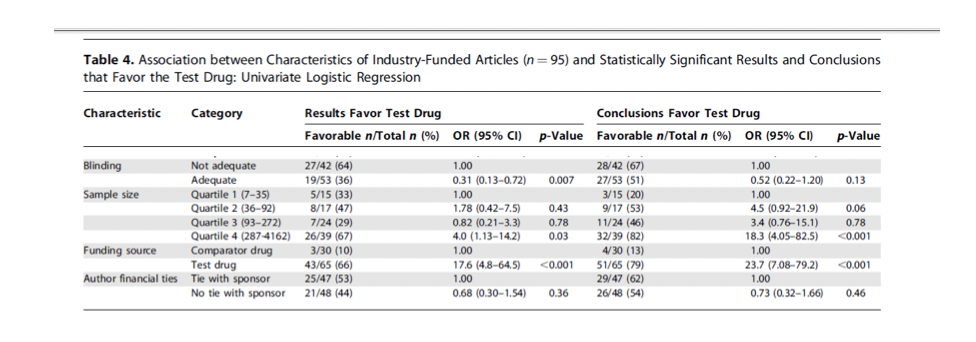
Of the 65 trials where the trial was sponsored by the maker of the
newer drug, favourable results were reported in 66% of the studies and
favourable conclusions drawn in 79% of the cases. Of the 30 studies
in which the trial sponsor was the maker of the older drug, only 3
(10%) reported favourable results for the non-sponsored drug (i.e. the
newer statins), and only 4 (13%) endorsed the non-sponsored drug in
the conclusion. The researchers concluded that ‘the main factor
associated with the results and conclusions of industry-sponsored
research… is research sponsorship.”
(See table 4 in the paper).
 Bero, L. (2007). Factors associated with findings of published trials of drug–drug comparisons: why some statins appear more efficacious than others. PLoS Medicine, 4(6), e184
Bero, L. (2007). Factors associated with findings of published trials of drug–drug comparisons: why some statins appear more efficacious than others. PLoS Medicine, 4(6), e184
Impact
A Cochrane systematic review comparing research sponsored industry found that treatment benefits were more likely to favour the sponsor’s products (relative risk = 1.27; 95% CI 1.17 to 1.37) and authors’ conclusions were more favourable (relative risk = 1.34; 95% CI 1.19 – 1.51).
Industry sponsorship was not shown to affect estimates of harm, but the quality of evidence on the subject is very low. Whether this also inflates the estimates for effect size (and if so to what degree) varies by drug. The observed differences could not be accounted for by standard measures for “risk of bias” assessments as these assess only study design features (related to internal validity).
One potential contributing factor is the selective publication of clinical trial results, with trials with positive results for a sponsor’s product more likely to be published than negative trials.
For example, Turner, (2008) found that 37 of 38 (97%) pre-market trials of antidepressants that the FDA judged to be positive were published. Of 36 trials with negative or questionable results, 22 (61%) were not published, 11 (31%) were published with narrative “spin”, and only 3 (8%) were accurately represented as negative.
As a result of the selective publication and other changes to the data analysis, the weighted average effect size in the published literature was inflated by roughly 30% compared to the data as submitted to the FDA.
The widespread use of antiarrhythmic drugs provides a more severe case. Class 1C antiarrhythmic drugs were prescribed to prevent cardiac arrest. Industry representatives and researchers working for industry persuaded the FDA to accept a surrogate outcome, despite concerns that the measure failed to account for the potential of the drug to cause harm (Holman, 2017). A large-scale RCT to test the drugs showed that they dramatically increased the risk of death (RR = 2.54). Before withdrawal, the widespread use of Class 1C antiarrhythmics was associated with tens of thousands of deaths (Moore, 1995).
Finally, review articles are also vulnerable to industry sponsorship bias. Barnes and Bero (1998) examined 106 survey articles that claimed to synthesise the existing literature on the health effects of second-hand smoke. Of these, 37% found that second-hand smoke was not associated with negative health consequences.
Barnes and Bero assessed whether each review article was peer-reviewed, whether authors had an affiliation with the tobacco industry, which topic the article focused on (e.g., heart disease, lung cancer, etc.), the year that the review was published, and the methodological quality of the review. The only factor associated with whether the review authors identified the negative effects of second-hand smoke was an affiliation with the tobacco industry. Of authors with no ties to industry, 87% (65/75) found negative health effects; whereas, only 6% (2/31) of authors with industry ties drew similar conclusions.
Reviews of the health effects of secondhand smoke funded by the tobacco industry were almost 90 times more likely to conclude that second-hand smoke was not harmful than reviews funded by other sources. This association was observed even when taking into account the methodological quality of the review articles.
Preventive steps
Some aspects of funding bias can be addressed pre-emptively by researchers retaining control over the design, conduct, analysis, and reporting of the study, especially avoiding research contracts which include non-disclosure agreements or that allow the sponsor to have any role in the design, conduct or publication of the research. Such steps are necessary but may not be sufficient to prevent funding bias, as the questions addressed by sponsored research may also help to shape results that are positive for the sponsor, and the impact of negative trial results on future funding opportunities may play a role.
The most effective preventative measure is likely a firewall, in which companies contribute to a general research fund but do not directly sponsor specific trials. Trials should be designed to serve scientific rather than marketing purposes. Comparators should be the best available product on the market and should include non-pharmacological interventions if relevant. Doses of the comparator drugs should be equivalent to the test drug. Detailed trial plans should be preregistered, including specification of planned secondary analyses. All data from the study should be publicly available. These interventions, which are becoming more common for drug studies, should be extended to include studies with other industry sponsors.

